Sep 27, 2019
Now you know it, I have degenerative arthritis of both knees. It has affected me for more than ten years. In 2017 because of progressive symptoms and limitation, I had thought that I would need to give up skiing, cycling and fly fishing. At the time, imaging was compatible with grade 4 OA at the patellofemoral part of the joint; but grade 2 to 3, between tibia and femur. My decision in late December of 2017 was a proprietary Platelet Rich Plasma filtered concentrate into both knees and it worked (growth factors). I skied, cycled and walked up spring creeks in Southwest Wisconsin all of 2018 and most of 2019 but now limited discomfort is recurring and my gait is changing. While as most persons over 65, there is some degree of wear and tear in the low back contributing to my discomfort after prolonged standing; the pain generator is inflammation from osteoarthritis of my knees. The question for me now is whether to repeat the procedure of 2017 that was successful in relieving pain and improving function by concentrating platelets and filtering the growth factor proteins in my plasma?
Fast forward to the end of this year or the beginning of 2020. The Personalized Stem Cell FDA application for The Clinical Trial using Adipose Derived Stem Cells to treat knee OA, Grade 2 to 4 has been approved as of late July of this year. As you may have learned, ours is the first center in the Country to have been trained and credentialed to recruit and perform the procedure (www.sheinkopmd.com and www.personalizedstemcells.com); several patients are now scheduled for the procedure and four more scheduled for the prescreening process. My personal issue is that I have OA affecting both knees. PSC has indicated it will be applying for FDA approval to treat patients with bilateral knee osteoarthritis sometime after mid-November. In order to assure my preparedness to ski in January and February of 2020 with full recovery and rehabilitation, I will need to have instituted treatment by December 1. As well, while the Wisconsin trout fishing season ends on October 15, it reopens about January 6. For those not familiar, the hiking, climbing, crawling and unstable walking in or along a spring creek can be as demanding as skiing down a mogul run on the ski slopes out West.
Here is my plan based on my stem cell experience, biologic familiarity, and privilege of having been selected as a Center of Excellence for the Personalized Stem Cell FDA approved Clinical Trial. If the FDA approves the PSC bilateral application by mid-November, I will opt for the latter. On the other hand, if my self-imposed recreational time table is in doubt of being kept, I will repeat the IRAP (PRP growth factor) interventions and then opt to proceed with the PSC undertaking when possible. To learn more about the Personalized Stem Cell Trial, call (847) 370-4523, To schedule an office visit, call (312) 475-1893
Good running, cycling, skiing, walking, golfing, boating, fly fishing, curling, bowling etc., etc., etc.
Jul 24, 2019
I am pleased to announce that our clinic is being considered for enrollment in the first of its kind stem cell clinical trial for knee arthritis.
Personalized Stem Cells, Inc. (PSC) has received approval from the FDA for conducting a clinical trial for treatment of knee arthritis with stem cells from a patients’ own fat. This is a tremendous honor since there is a limit of ten clinics around the country in the clinical trial. Our practice and our staff were selected by PSC due to our leadership and experience in treatment of orthopedic conditions, and our experience with regenerative medicine.
The clinical trial includes treatment, follow up, and cell storage for potential use in the future if, or when, additional treatments are allowed by the FDA. PSC has FDA inspected facilities, approved processing procedures, quality testing procedures, and cell storage and shipping procedures. More information about the clinical trial can be found HERE.
Our office will be receiving additional information in the near future regarding the details of the clinical trial.
Please contact us at 312-475-1893
Call 312-475-1893 if you are interested in being considered for this study and we will put you on the list for when we are able to start formal study recruitment and screening.
Click here to read the press release.
Apr 4, 2019
A patient presents to the office because of pain in the knee with or without a history of injury. An examination is performed followed by an X-Ray. Osteoarthritis may or may not be seen on the X-ray. If there is an altered range of knee motion when compared to the “normal” side, then a preexisting condition is considered. Whether or not the physician considers arthritis, an MRI is requested. The MRI report 48 hours after imaging is consistent with a torn medial meniscus. Should all patients with a torn medial meniscus undergo surgical intervention? If surgery is undertaken, should the procedure be a repair or a partial removal? The management of meniscal injuries must be influenced by the knowledge that meniscal integrity is important in load distribution across the joint. Meniscal injury causes altered joint mechanics and is related to the onset of arthritis.
According to a recently published online article in the British Journal of Sports Medicine, arthroscopic partial meniscectomy (APM) may not be the best option for all patients with knee pain and meniscal tear. Researchers investigated patients with meniscal tears that compared Arthroscopic Partial Meniscectomy to nonsurgical intervention, pharmacological intervention, and no intervention. At six to 12 months, APM patients had a slight improvement in knee pain, knee-specific quality of life, and knee function compared to physiotherapy patients. When excluding osteoarthritis (OA) patients, the aforementioned outcomes exhibited small to moderate improvement. Knee pain, function, and quality of life did not improve for APM patients compared to placebo surgery patients at six to 12 months regardless of OA status.
There may, however, be a small-to-moderate benefit from APM compared with physiotherapy for patients without osteoarthritis and who have mechanical or obstructive signs. Arthroscopic partial meniscectomy (APM), a keyhole surgery where loose and fragmented pieces of a torn meniscus is removed, is one of the most common orthopedic procedures performed. Over half of these are performed to treat a meniscus tear in a degenerative knee; however, several recent randomized trials have shown that Arthroscopic Partial Meniscectomy is not superior to conservative treatment or placebo treating meniscus tears associated with a degenerative knee. On the other hand, there is universal agreement that the traumatic meniscus tear, the result of a knee injury in a younger patient with otherwise healthy knee (with no degeneration), should be treated by surgery.
Then what is the downside of meniscal injury and surgery? The medial and lateral meniscus together provide shock absorption, establish a broad base of contact surface and help provide stability to the knee. Those who have undergone total or partial meniscectomy should understand that in five to 15 years, they will develop degenerative arthritis. The long-term outcomes of those whose tears were treated by repair rather than removal has not been established. My Regenerative Medicine practice in part, is the result of those seeking to postpone or avoid a Total Knee Replacement years after a meniscal injury followed by arthroscopic surgery. As long as the arthritis has not progressed to a Grade 4, I am able to assist the patient with joint restoration, at times joint regeneration, it is matter of age and health. While I am able to offer joint restoration, on occasion, joint restoration for those who sustained meniscal and Anterior Cruciate injury in the past, is there anything that could be used as an adjunct at the time of the meniscal injury to promote healing without surgery or postpone, perhaps avoid future postraumatic arthritis?
To learn more. Schedule a consultation (312) 475-1893.You may view my web site at www.sheinkopmd.com.
Tags: ACL, ACL Injury, anterior cruciate, arthritic knee, arthritis, Autologous Protein Concentrate, baseball, BMC, board-certified, Bone Marrow Concentrate, bone marrow edema, cells, cellular orthopedic, cellular orthopedics, FDA, football, golf, Growth Factors, hematopoietic cell, injection, Interleukin 1 Receptor Antagonist Protein, IRAP, joint health, joint pain, knee replacement, lipogems, meniscal injury, meniscectomy, Mesenchymal Stem Cell, micro-fragmented adipose, muscle injury, muscle strain, OA, Orthopedic Surgeon, Osteoarthritis, pain, Physical Therapy, Platelet Rich Plasma, platelets, PRP, regenerative medicine, repair, Rotator cuff tear, soccer, sports injuries, sports medicine, stem cells, strain, tear, torn medial meniscus, training, volleyball
Oct 5, 2018
After reviewing a CT scan of the right knee where a Total Knee Replacement had been done provided by a patient who sought my opinion for stem cell treatment of her arthritic left knee, I called her to discuss the possible reasons that the right Total Knee Replacement that she had undergone over ten years ago was never satisfactory. At the time of the right TKR for arthritis, she had elected for what seemed at the time as a logical intervention for a painful, arthritic, right knee. The outcome was at first complicated by a lot of postoperative swelling and pain; at one-year post-operative, the patient had never been pain-free nor had she ever regained her preoperative range of motion and that adverse outcome has persisted for over ten years. Fast forward a decade and the right knee has continued to be a source of pain and limited motion; so much so that the patient decided to seek consultation for a Stem Cell treatment for her now arthritic left knee.
Her first question was basically “does stem cell therapy for knees work?”. Our data collected on patients treated in this office over the past six years, since I started my practice, is compatible with an 85% patient satisfaction rate. We have not recorded one complication. To be forthright, not every patient has a perfect result; but the vast majority experience marked reduction in pain, increased motion and a significant improvement in functional capacity. Unlike a failed outcome of a Total Knee Replacement, our stem cell injections may be repeated at anytime if the benefits of the initial intervention do not last.
When it comes to the cost of a stem cell intervention, while the Total Joint Replacement is more often than not covered by some type of insurance, our fee schedule is in keeping in line with the deductible a patient has to pay out of pocket for the major surgery. If you do the math and additionally compare the risks and benefits of a stem cell intervention with a total knee replacement, you will note:
- Remote to little risk of complication with a stem cell procedure
- Comparable cost comparing my charges for a stem cell intervention with the inherent deductible obligation for a Total Joint Replacement
- Rapid recovery with a stem cell intervention versus the risks of an adverse outcome as experienced by the patient seeking consultation for stem cells
To seek consultation call (312) 475-1893. You may visit my website at WWW.sheinkopmd.com
Tags: cellular orthopedics, ct scan, joint health, joint replacement, knee pain, knee replacement, Osteoarthritis, regenerative medicine, stem cell treatment, TKA
Jul 19, 2018
The argument frequently advanced by orthopedic surgeons in response to a patient’s inquiry concerning stem cells for arthritis is that it is too early, there is not enough research, It is better to have a major surgical procedure. For those of you who have read my blog or have sought orthopedic consultation in my office, I have emphasized that my recommendations are evidence based. Each patient, for whom I have completed a cellular orthopedic intervention for arthritis, has been entered into a registry or clinical outcomes data base, IRB approved. Just as I pioneered the integration of clinical care with clinical research over 37 years as a joint replacement surgeon, so too do I now partake in the growth and development of the clinical pathways for regenerative medicine.
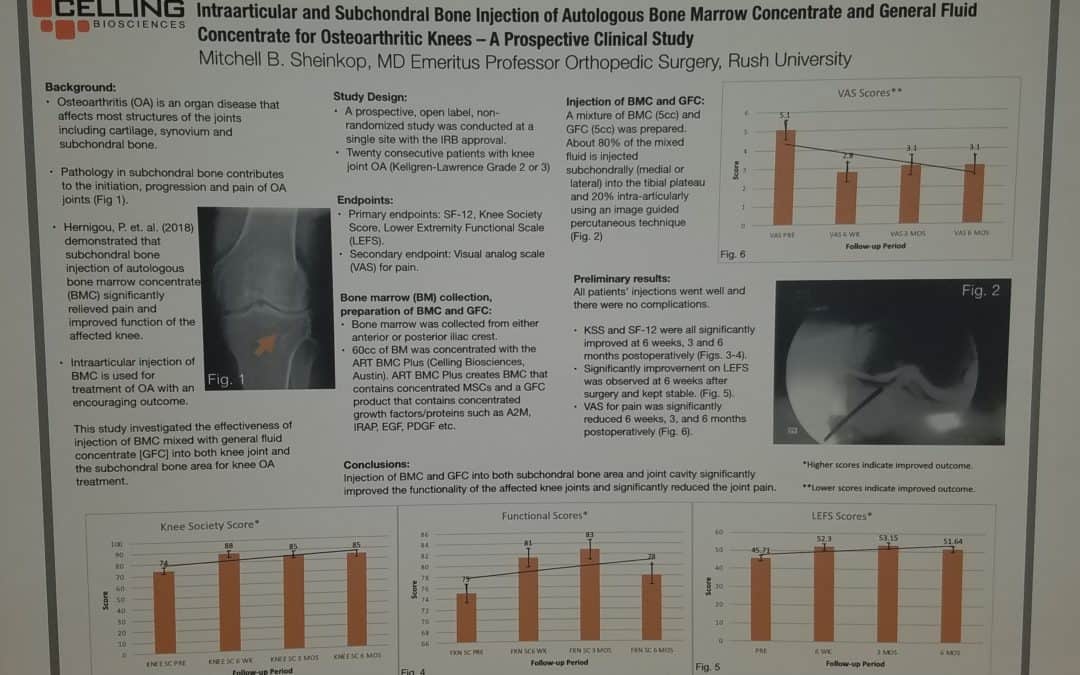
Last month, I exhibited a poster at a large regenerative medicine meeting wherein I shared my preliminary outcomes and thus educated other professionals using Intraarticular and Subchondral Bone Injection of Autologous Bone Marrow Concentrate and General Fluid Concentrate for Osteoarthritic Knees-A Prospective Clinical Study. Osteoarthritis is an organ disease that affects most structures of joints including cartilage, synovium and subchondral bone. Pathology in subchondral bone contributes to the initiation, progression and pain of Osteoarthritis. In previous European studies, the injection of autologous bone marrow concentrates into bone supporting the joint significantly relieved pain and improved function of the affected knee. The preliminary outcomes in the study that I presented via a poster exhibit, investigated the effectiveness of injections of Bone Marrow Concentrate with General Fluid Concentrate (Growth factors), into both the knee joint and the subchondral bone. The study recorded all the standard Endpoints I had previously used in joint replacement clinical outcomes trials.
Bone Marrow was collected from the pelvis and a filtration system allowed for concentration of Mesenchymal Stem Cells, Platelets, Precursor Cells and Growth factors such as A2M, IRAP, EGF, PDGF, TNF-B blocker, etc. After preparation, a mixture of Bone Marrow Concentrate and Growth factor Concentrate was injected into the bone (subchondral) and into the joint.
In the study, all patient injections went well and there were no complications. The Preliminary Results documented diminished pain and improved function. We concluded that injection of Bone Marrow Concentrate and Growth factor Concentrate into both the subchondral bone area and joint cavity significantly improved function of the affected knee joints and significantly reduced joint pain. While there are many stem cell providers to be found because of their marketing, choose the center of excellence in Cellular Orthopedics that is evidence based.
Call to schedule a scientific based consultation from an orthopedic surgeon 1 (312) 475-1893.
You may access my web site at www.SheinkopMD.com.
Tags: avascular necrosis, bone lession, bone marrow, Cartilage, cellular orthopedics, clinical study, Growth Factors, IRAP, joint pain, joint replacement, knee pain, knee replacement, meniscus tear, Osteoarthritis, platelets, PRP, regenerative medicine, sports medicine, stem cells, subchondral bone
Apr 26, 2018
On Wednesday, I completed several bone marrow concentrate procedures for patients with arthritic knees. You will recall that Concentrated Bone Marrow contains living Adult Mesenchymal Stem Cells, Growth Factors, Platelets, Exosomes, Precursor Cells and more allowing for pain relief, improved function and possible regeneration in those afflicted by arthritis. In the afternoon, four patients underwent Autologous Platelet and Growth Factor interventions; two in the hip and two in their knees. An example of the outcome, now four months following intervention in my own knees and hips, I spent last weekend hiking along several spring creeks, fly fishing in Southwest Wisconsin in the morning and planting over 150 Lilly bulbs in the afternoon. Admittedly, I slept well on Saturday and Sunday night but visited the health club on these past Tuesday and Wednesday evenings for my fitness routines.
On Monday, we finalized and edited a manuscript reporting the results of 56 patients with arthritic knees, followed for 2 to 4 years having received Bone Marrow Concentrate. Using the same outcome metrics and statistical tabulation methods I had employed as a joint replacement surgeon, this study is one of the most significant trials ever completed and to be published in Cellular Orthopedics. Our study not only will help determine the indications for a “stem cell” procedure, but also assist in determining how long the benefits will last, and provide a road map for when adjunct or repeat interventions are indicated. Now the physician will be better prepared to help a patient decide between a Total Joint Replacement and a Cellular Orthopedic intervention on an evidence based knowledge.
I am writing this Blog while flying to San Jose, California where I am partaking in advanced training that will allow me to expand my regenerative medicine practice to the low back. Again and again, patients ask as to what I might offer to address low back pain and disc disease now that I have successfully intervened in an arthritic hip or knee. Indications and techniques for addressing the lumbar spine will make up the curriculum enabling me to add discogenic and degenerative arthritic conditions of the low back to my scope of regenerative care by mid May.
To the patient who called, “I heard through the grapevine that it doesn’t work”, you may avoid falling victim to the Fake Stem Cell claims in newspaper ads or via celebrity testimonials; those in amniotic fluid are dead on arrival to you. Seek scientific evidence at (312) 475-1893 or learn more on my web site where you may watch my webinar www.Ilcellulartherapy.com.
Tags: Adult Mesenchymal Stem Cells, arthritic conditions, back pain, cellular orthopedics, Concentrated Bone Marrow, degenerative disc disease, discogenic, Exosomes, Growth Factors, Hip pain, joint pain, knee pain, Osteoarthritis, platelets, Precursor Cells, Regenerative Pain Center, shoulder pain, stem cell therapy


