
Oct 19, 2020
There are two clinical, readily available sources of adult mesenchymal stem cells, to the cellular orthopedist; the musculoskeletal building cells that have the potential to stop the progression of arthritis. These cells may reverse the damage, regenerate cartilage, and eliminate the pain generator, inflammation. MSCs work in conjunction with platelets and proteins called Growth Factors.
In my clinical evidence-based setting, we have explored and continue to investigate the several possible approaches to treating the arthritic joint with either your adult mesenchymal stem cells, your platelets and /or your growth factors either separately or in combination with each other. I have previously authored or co-authored the results of the clinical outcomes of using adipose derived approaches to arthritis and bone marrow concentrate containing stem cells in restoring the arthritic joint to well-being. My most recent scientific publication is based on using concentrated bone marrow both in the joint and in the subchondral bone adjacent to the joint as there is increasing evidence regarding the role of the subchondral bone in the causation of arthritis.
Mesenchymal Stem Cell Treatment Clinical Trial Update
This past Thursday night, the several of us involved in the first FDA approved, recently completed, Clinical Stem Cell Trial (Personalizedstemcells.com.), reviewed the preliminary responses in 38 enrolled patients. First and foremost, we recorded no serious adverse events; no complications from the drug injected after liposuction and preparation. Several minor complaints were observed as part of the liposuction process. Of the 38 patients, the vast majority are enjoying a positive response in the treated arthritic joint. The FDA has required our monitoring the patients for a year; however, our next milestone follow-up will be at day 84 from the time of the joint injection.
Based on what we have learned to date about the safety and efficacy of adipose derived stem cells in the treatment of the arthritic knee, our investigators under the auspices of PersonalizedStemCells.com will be applying for a second phase clinical trial at the knee; but additionally, safety and efficacy for the hip and for several joints at the same time. I will announce the start of trial enrollment when approved in this blog and on my website, www.sheinkopmd.com.
These new clinical trials probably will not be open to enrollment until December or perhaps the first quarter of 2021. For those patients who are seeking relief now without jeopardizing participation in the clinical trial, call (312) 475-1893. I offer a full menu of biologic interventions for the arthritic joint including concentrated bone marrow, Proprietary Platelet Rich Plasma (PRP), Growth Factors and other Orthobiologics and determine which is the best option at the time of the office visit
Tags: cellular therapy, clinical study, clinical trial updates, stem cell clinical trials, stem cell treatments
Jul 19, 2018
The argument frequently advanced by orthopedic surgeons in response to a patient’s inquiry concerning stem cells for arthritis is that it is too early, there is not enough research, It is better to have a major surgical procedure. For those of you who have read my blog or have sought orthopedic consultation in my office, I have emphasized that my recommendations are evidence based. Each patient, for whom I have completed a cellular orthopedic intervention for arthritis, has been entered into a registry or clinical outcomes data base, IRB approved. Just as I pioneered the integration of clinical care with clinical research over 37 years as a joint replacement surgeon, so too do I now partake in the growth and development of the clinical pathways for regenerative medicine.
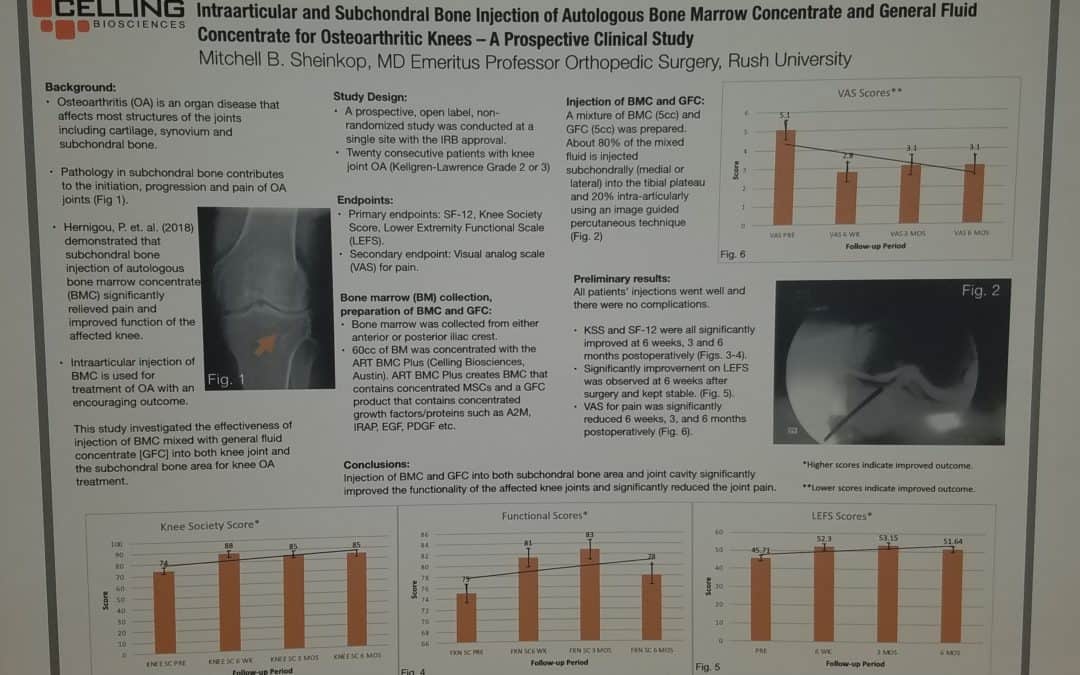
Last month, I exhibited a poster at a large regenerative medicine meeting wherein I shared my preliminary outcomes and thus educated other professionals using Intraarticular and Subchondral Bone Injection of Autologous Bone Marrow Concentrate and General Fluid Concentrate for Osteoarthritic Knees-A Prospective Clinical Study. Osteoarthritis is an organ disease that affects most structures of joints including cartilage, synovium and subchondral bone. Pathology in subchondral bone contributes to the initiation, progression and pain of Osteoarthritis. In previous European studies, the injection of autologous bone marrow concentrates into bone supporting the joint significantly relieved pain and improved function of the affected knee. The preliminary outcomes in the study that I presented via a poster exhibit, investigated the effectiveness of injections of Bone Marrow Concentrate with General Fluid Concentrate (Growth factors), into both the knee joint and the subchondral bone. The study recorded all the standard Endpoints I had previously used in joint replacement clinical outcomes trials.
Bone Marrow was collected from the pelvis and a filtration system allowed for concentration of Mesenchymal Stem Cells, Platelets, Precursor Cells and Growth factors such as A2M, IRAP, EGF, PDGF, TNF-B blocker, etc. After preparation, a mixture of Bone Marrow Concentrate and Growth factor Concentrate was injected into the bone (subchondral) and into the joint.
In the study, all patient injections went well and there were no complications. The Preliminary Results documented diminished pain and improved function. We concluded that injection of Bone Marrow Concentrate and Growth factor Concentrate into both the subchondral bone area and joint cavity significantly improved function of the affected knee joints and significantly reduced joint pain. While there are many stem cell providers to be found because of their marketing, choose the center of excellence in Cellular Orthopedics that is evidence based.
Call to schedule a scientific based consultation from an orthopedic surgeon 1 (312) 475-1893.
You may access my web site at www.SheinkopMD.com.
Tags: avascular necrosis, bone lession, bone marrow, Cartilage, cellular orthopedics, clinical study, Growth Factors, IRAP, joint pain, joint replacement, knee pain, knee replacement, meniscus tear, Osteoarthritis, platelets, PRP, regenerative medicine, sports medicine, stem cells, subchondral bone

