
Nov 24, 2020
Can Activity Bolster Cartilage?
It may even delay arthritis and influence cartilage healing. Could running actually be good for your knees, hips, and back? Cartilage, which doesn’t have a blood supply of its own, is generally thought to not have the ability to repair itself once damaged. Yet, in real life, while some some runners will develop knee arthritis, as a cohort, runners are statistically less likely to experience arthritis of the knee, hip or back than non-runners. In recent studies, animals that ran had thicker, healthier cartilage than those that were sedentary, suggesting that the active animals’ cartilage had changed in response to running. Perhaps in humans, cartilage in runners likewise might adapt to repetitive loading cycles. Taking it a step further, you might presume that the cartilage will grow thicker and remodel just as muscle does when we exercise.
Looking at the possible pathways that cartilage uses to remodel and heal in adults with activity, might the explanation be biologics found in your bone marrow, adipose tissue, and plasma? Assume that you have addressed your joint and back pain with the usual and customary measures of Non-Steroidal Anti-inflammatory Medications; weight reduction; Physical Therapy; as well as braces, orthotics and supports; and you still are experiencing symptoms and functional impairment. Next you have tried injections with hyaluronic acid or cortisone with diminishing return; in the latter case with potential damage to your cartilage. The underlying source for joint and back pain are molecules resulting from inflammation in those joints. These molecules are enzymatic proteins that generate pain and destroy the cartilage cells and tissues in the joint or disc space.
Activities That Can Help
Our bodies do produce defense mechanisms against destructive enzymes; but in order to be effective, these proteins and molecules must get into the joint or disc space in high concentrations. Cyclic loading by activities such as running or cycling is a mechanical mechanism by which your body’s growth factors, stem cells and the like are able to effect cartilage in a joint or intervertebral disc; think of it as a sponge like action of absorption. Another means of delivering the Growth Factor Protein and Stem Cell is via injection following my harvesting your platelets, plasma, circulating blood, adipose tissue or bone marrow; concentrating and filtering; and then injection into the arthritic joint or failing intervertebral disc. The injection of biologics may provide the necessary concentration of molecules to bind and remove destructive enzymes from you joints as well as initiate a healing process.
To learn more about Orthobiologics, visit my website at www.sheinkopmd.com. You may schedule an appointment by calling (312) 475-1893
Tags: Cartilage, cartilage regeneration, cell based therapies, cellular therapy, growth and repair

Mar 22, 2019
I will let the scientific facts speak for themselves. Keep this in mind the next time you see the advertisement from the Stem Cell hustlers of America. There is no such thing as a free lunch.
From: The American Journal of Sports Medicine
Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells with Bone Marrow Comparison
Alberto J. Panero, DO*, Alan M. Hirahara, MD, FRCSC, Wyatt J. Andersen, ATC,
First Published 7, 2019 Research Article https://doi.org/10.1177/0363546519829034
Abstract
Background:
In vivo amniotic fluid is known to contain a population of mesenchymal stem cells (MSCs) and growth factors and has been shown to assist in healing when used as an adjunct in procedures across multiple medical specialties. It is unclear whether amniotic fluid products (AFPs) contain MSCs and, if so, whether the cells remain viable after processing.
Purpose: To determine whether MSCs, growth factors, and hyaluronan are present in commercially available Amniotic Fluid Products.
Study Design:
Descriptive laboratory study.
Methods:
Seven commercial companies that provide amniotic fluid were invited to participate in the study; 3 companies (the manufacturers of PalinGen, FloGraft, and Genesis AFPs) agreed to participate and donated AFPs for analysis. The AFPs were evaluated for the presence of MSCs, various growth factors relevant to orthopaedics (platelet-derived growth factor ββ, vascular endothelial growth factor, interleukin 8, bone morphogenetic protein 2, transforming growth factor β1), and hyaluronan by enzyme-linked immunosorbent assay and culture of fibroblast colony-forming units. These products were compared with unprocessed amniotic fluid and 2 separate samples of MSCs derived from human bone marrow aspirates. All groups used the same culture medium and expansion techniques. Identical testing and analysis procedures were used for all samples.
Results:
MSCs could not be identified in the commercial AFPs or the unprocessed amniotic fluid. MSCs could be cultured from the bone marrow aspirates. Nucleated cells were found in 2 products (PalinGen and FloGraft), but most of these cells were dead. The few living cells did not exhibit established characteristics of MSCs. Growth factors and hyaluronan were present in all groups at varying levels.
Conclusion:
The Amniotic Fluid Products studied should not be considered “stem cell” therapies, and researchers should use caution when evaluating commercial claims that products contain stem cells. Given their growth factor content, however, AFPs may still represent a promising tool for orthopaedic treatment.
Clinical Relevance:
Amniotic fluid has been proposed as an allogenic means for introducing MSCs. This study was unable to confirm that commercial AFPs contain MSCs.
Tags: amniotic fluid, arthritis, Bone Marrow Concentrate, Cartilage, cellular orthopedics, growth factors/healing enhancement, joint pain, mesenchymal stem cells, MSC, Orthopedic Surgeon, Osteoarthritis, regeneration, sports medicine, stem cell therapy
Nov 8, 2018
My goal is to inform each and every patient who presents with a painful joint, the cause of their pain; and based on our scientific and clinical evidence, that intervention which will have the greatest chance of short term and long-term success. While inflammation in the joint is a proximate cause of pain, that pain is not generated by cartilage deterioration as cartilage doesn’t have a nerve supply. While joint pain in part is generated by the synovial tissue lining the arthritic or traumatized joint, the subchondral bone supporting the joint may be even more important when it comes to the pain and limitations resulting from the arthritic affliction.
Bone pathologies resulting from acute or chronic injury presenting as bone marrow lesions associated with insufficiency fractures, persistent bone bruises, osteoarthritis and early stages of avascular necrosis are too often neglected by those holding themselves out to be regenerative medicine specialists. Options for the treatment of these subchondral conditions require a core decompression of the problematic bone and direct application of either bone marrow aspirate or a synthetic orthobiologic. The biologic treatment of bone marrow lesions with these techniques that encourage physiologic bone remodeling and repair when combined with Stem Cell and Protein/Growth Factor concentrates into an arthritic joint offers the best chance for joint preservation and a successful outcome for the patient undergoing a Stem Cell procedure.
Are there Stem Cells in Cord Blood, Wharton’s Jelly or Amniotic Fluid? These three alleged sources of Stem cells are processed when collected. The tissues are then cryopreserved with DMSO or some other cryopreservant. When thawing takes place, the few cells contained do not survive the thawing process. Additionally, DMSO is cytotoxic, a cell killer at room temperature.
As many of my patients are aware, I began my Cellular Orthopedic journey some years ago as an early member of the Regenexx Network. While my personal and practice ethos as the only orthopedic surgeon caused me to leave the network, I still follow the Blog and I find the one posted today most appropriate.

I was on a local radio show this week and a woman called in and claimed that she had been defrauded by a local chiropractic clinic. She paid big bucks for what she was told were “millions of young stem cells” injected intravenous. As I will show you this morning, as a medical expert in this area, I can show you that she is more likely than not the victim of consumer fraud. Let me explain.
The Problem of the Chiro Clinic Bait and Switch
I’ve blogged extensively about how chiropractic, acupuncture, naturopathic, and some physician clinics are defrauding patients by claiming to inject millions of live and young stem cells from amniotic fluid or cord blood (or other products). The problem is that none of these 361 registered tissue products has any significant number of live stem cells.
Tags: ACL Injury, bone edema, bone lesion, bone marrow, Cartilage, chondromalacia, chronic pain, Cord blood, cryopreserved, Hip, joint pain, joint replacement, Knee, meniscus tear, naturopathic, Osteoarthritis, osteochondral defect, regenerative medicine, shoulder, stem cell, subcondral bone
Oct 18, 2018
We are speaking of stem cell therapy integrated with clinical research, and the resultant evidence-based stem cell intervention. Osteoarthritis is becoming more prevalent as I am seeing younger patients with arthritis as a consequence of sporting injuries such as ACL tears. The baby boomer population is experiencing accelerated onset of arthritis; their joints are prematurely aging in large numbers. At the same time, the master population is aging and living longer. As a result, I continually research biologic interventions to best address the ever-increasing number of those effected.
Why should a patient choose an orthopedic surgeon to manage their Osteoarthritic related symptoms and functional impairment? Our world is evidence based.
Study Observes Better Outcomes for OA Patients Treated by an Orthopaedic Specialist
In a retrospective study published online in BMC Musculoskeletal Disorders, shoulder osteoarthritis (OA) patients received faster and more invasive treatment when they received a new diagnosis from an orthopaedic specialist (OS) versus a nonorthopaedic physician (NOP). Patients with shoulder OA (n = 572) received care from either an OS (n = 474) or NOP (n = 98) on the date of their index shoulder visit. OS patients received their first treatment significantly quicker than the NOP cohort (16.3 days versus 32.3 days, respectively). The OS group also had higher rates of operative treatment within one year following their initial visit.
Study: Patients Report Similar Improvements for Nonobstructive Meniscal Tear with PT and Early Surgery
Physical therapy (PT) may not be inferior to early operative treatment of arthroscopic partial meniscectomy (APM) for improving knee functionality in patients with nonobstructive meniscal tears, according to a study published online inJAMA. The randomized clinical trial included 321 patients with nonobstructive meniscal tears aged 45 to 70 years who were treated at nine hospitals in the Netherlands between July 17, 2013, and Nov. 4, 2015. Patients were treated with APM (n = 159) or a predefined PT protocol (n = 162) that included 16 exercise therapy sessions over eight weeks. PT sessions focused on coordination and closed kinetic chain strength exercises. At 24-month follow-up, knee functionality in the PT group improved by 20.4 points compared to 26.2 points in the APM group. The difference did not exceed the noninferiority margin.
In order to maximize the benefits, Orthobiologics, that is stem cell therapy must be integrated with clinical research, and the resultant evidence-based stem cell intervention followed long term. In my practice, I am researching biologic interventions to address the ever-increasing number of those effected, not one and done. To learn more or schedule a consultation, Call (312)475-1893. You may visit my web site and read my blogs at www.sheinkopmd.com
Tags: ACL tear, arthritis, Cartilage, cellular orthopedic, joint pain, joint replacement, knee pain, MCL tear, meniscus tear, menisectomy, orthobiologics, orthopedic surgen, Osteoarthritis, Physical Therapy, PRP, regenerative medicine, stem cell
Jul 19, 2018
The argument frequently advanced by orthopedic surgeons in response to a patient’s inquiry concerning stem cells for arthritis is that it is too early, there is not enough research, It is better to have a major surgical procedure. For those of you who have read my blog or have sought orthopedic consultation in my office, I have emphasized that my recommendations are evidence based. Each patient, for whom I have completed a cellular orthopedic intervention for arthritis, has been entered into a registry or clinical outcomes data base, IRB approved. Just as I pioneered the integration of clinical care with clinical research over 37 years as a joint replacement surgeon, so too do I now partake in the growth and development of the clinical pathways for regenerative medicine.
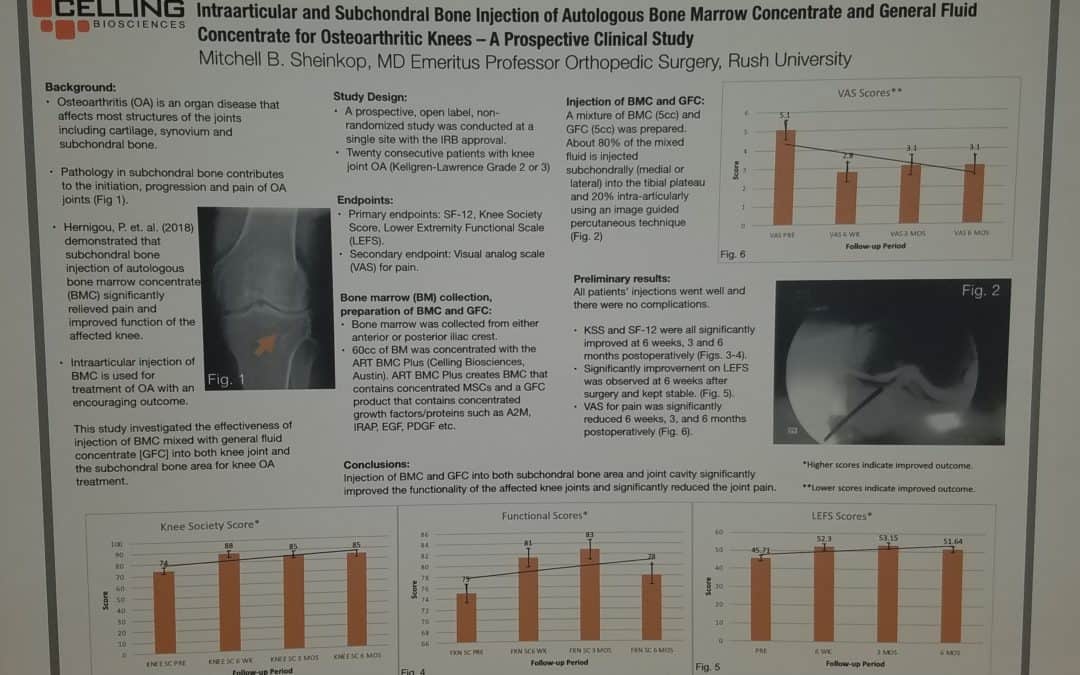
Last month, I exhibited a poster at a large regenerative medicine meeting wherein I shared my preliminary outcomes and thus educated other professionals using Intraarticular and Subchondral Bone Injection of Autologous Bone Marrow Concentrate and General Fluid Concentrate for Osteoarthritic Knees-A Prospective Clinical Study. Osteoarthritis is an organ disease that affects most structures of joints including cartilage, synovium and subchondral bone. Pathology in subchondral bone contributes to the initiation, progression and pain of Osteoarthritis. In previous European studies, the injection of autologous bone marrow concentrates into bone supporting the joint significantly relieved pain and improved function of the affected knee. The preliminary outcomes in the study that I presented via a poster exhibit, investigated the effectiveness of injections of Bone Marrow Concentrate with General Fluid Concentrate (Growth factors), into both the knee joint and the subchondral bone. The study recorded all the standard Endpoints I had previously used in joint replacement clinical outcomes trials.
Bone Marrow was collected from the pelvis and a filtration system allowed for concentration of Mesenchymal Stem Cells, Platelets, Precursor Cells and Growth factors such as A2M, IRAP, EGF, PDGF, TNF-B blocker, etc. After preparation, a mixture of Bone Marrow Concentrate and Growth factor Concentrate was injected into the bone (subchondral) and into the joint.
In the study, all patient injections went well and there were no complications. The Preliminary Results documented diminished pain and improved function. We concluded that injection of Bone Marrow Concentrate and Growth factor Concentrate into both the subchondral bone area and joint cavity significantly improved function of the affected knee joints and significantly reduced joint pain. While there are many stem cell providers to be found because of their marketing, choose the center of excellence in Cellular Orthopedics that is evidence based.
Call to schedule a scientific based consultation from an orthopedic surgeon 1 (312) 475-1893.
You may access my web site at www.SheinkopMD.com.
Tags: avascular necrosis, bone lession, bone marrow, Cartilage, cellular orthopedics, clinical study, Growth Factors, IRAP, joint pain, joint replacement, knee pain, knee replacement, meniscus tear, Osteoarthritis, platelets, PRP, regenerative medicine, sports medicine, stem cells, subchondral bone





