
Oct 11, 2018
In general, a Growth Factor is a term used to describe a protein produced be a variety of different cell types that binds to specific receptors on different cellular surfaces. They may on the one hand stimulate cell growth; on the other hand, some growth factors may block actions on target cells of a different protein. Cytokines are another class of signaling proteins more closely related to hormones. The subject matter is quite complex; but, I wanted to introduce the subject of Growth Factors into my Blog as growth factors are increasingly gaining attention in combating and reversing the progression of arthritis.
Already in use is Tissue Necrosis Factor-alpha blocker, frequently prescribed in a proprietary form as Humera, for inflammatory arthritis and arthropathy such as Rheumatoid Arthritis, Psoriatic Arthropathy and Ankylosing Spondylitis. It works by blocking a protein (TNF-Alpha), found in the body’s immune system and responsible for joint swelling and inflammation. By so doing, Humera reduces symptoms, prevents bone and cartilage damage and improves physical function.
Outside of the United States, Interleukin 1 Receptor Antagonist Protein (IRAP) is used for the symptoms of grades 2 and 3 Osteoarthritis by binding to the cell surface and preventing IL-1 from sending a proinflammatory message to that cell. IRAP is currently the basis for a clinical trial taking place in the United States to document its safety and efficacy. The sponsors of the trial hope for full enrollment and outcomes analysis in the not too distant future; so, the Growth Factor might be used with FDA approval in clinical practice. It was for IRAP that one-time basketball great Kobe Bryant, travelled to Dusseldorf Germany, ten years ago with grade 4 osteoarthritis of the knee thereby prolonging his career by six years.
On Tuesday of this week, I received a call form a company exploring a safety and feasibility trial for yet another growth factor approach with the latter derived from another human biologic resource. Last March, there was a contact from a global pharmaceutical company asking my help in developing a trial for yet another type of a growth factor approach in dealing with the symptoms and limitations of arthritis. I introduce the Growth Factor subject matter now to better update my reader as well as prepare you for the coming debate that Growth Factors are equal to; perhaps more important as we age than stem cells for dealing with the symptoms, functional limitations and progression of arthritis.
To learn more, you may call for a consultation. (312) 475-1893 or visit my web site at WWW.Sheinkopmd.com
Tags: autologous, bone marrow, Growth Factors, Interleukin 1 Receptor Antagonist Protein, IRAP, joint pain, OA, Osteoarthritis, PRP, stem cells
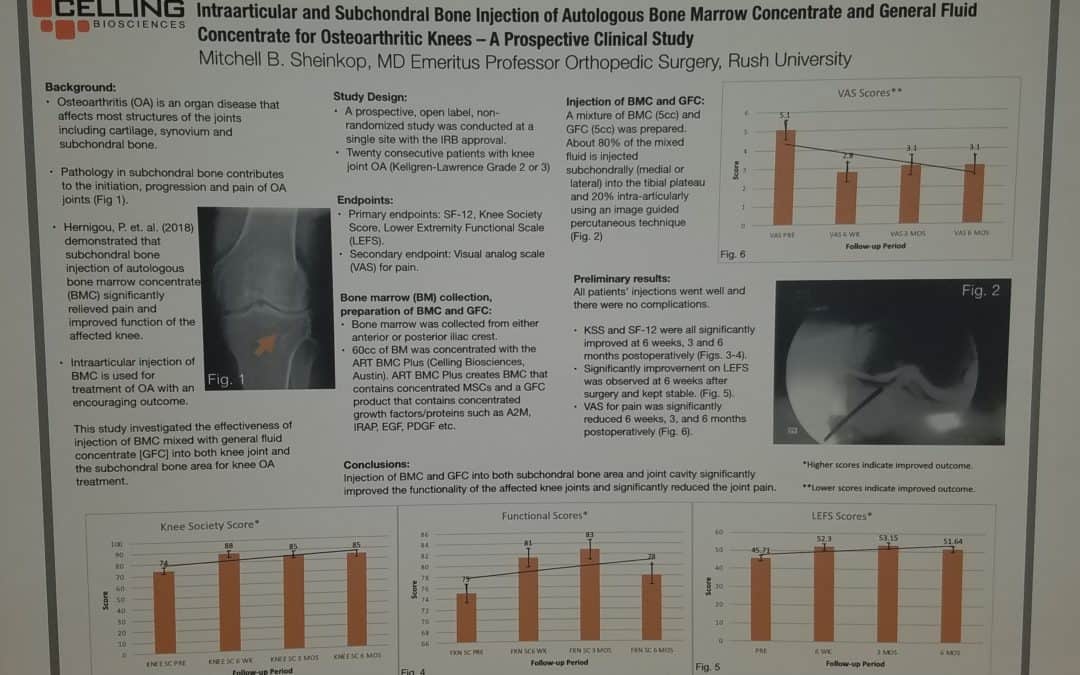
Jul 19, 2018
The argument frequently advanced by orthopedic surgeons in response to a patient’s inquiry concerning stem cells for arthritis is that it is too early, there is not enough research, It is better to have a major surgical procedure. For those of you who have read my blog or have sought orthopedic consultation in my office, I have emphasized that my recommendations are evidence based. Each patient, for whom I have completed a cellular orthopedic intervention for arthritis, has been entered into a registry or clinical outcomes data base, IRB approved. Just as I pioneered the integration of clinical care with clinical research over 37 years as a joint replacement surgeon, so too do I now partake in the growth and development of the clinical pathways for regenerative medicine.
Last month, I exhibited a poster at a large regenerative medicine meeting wherein I shared my preliminary outcomes and thus educated other professionals using Intraarticular and Subchondral Bone Injection of Autologous Bone Marrow Concentrate and General Fluid Concentrate for Osteoarthritic Knees-A Prospective Clinical Study. Osteoarthritis is an organ disease that affects most structures of joints including cartilage, synovium and subchondral bone. Pathology in subchondral bone contributes to the initiation, progression and pain of Osteoarthritis. In previous European studies, the injection of autologous bone marrow concentrates into bone supporting the joint significantly relieved pain and improved function of the affected knee. The preliminary outcomes in the study that I presented via a poster exhibit, investigated the effectiveness of injections of Bone Marrow Concentrate with General Fluid Concentrate (Growth factors), into both the knee joint and the subchondral bone. The study recorded all the standard Endpoints I had previously used in joint replacement clinical outcomes trials.
Bone Marrow was collected from the pelvis and a filtration system allowed for concentration of Mesenchymal Stem Cells, Platelets, Precursor Cells and Growth factors such as A2M, IRAP, EGF, PDGF, TNF-B blocker, etc. After preparation, a mixture of Bone Marrow Concentrate and Growth factor Concentrate was injected into the bone (subchondral) and into the joint.
In the study, all patient injections went well and there were no complications. The Preliminary Results documented diminished pain and improved function. We concluded that injection of Bone Marrow Concentrate and Growth factor Concentrate into both the subchondral bone area and joint cavity significantly improved function of the affected knee joints and significantly reduced joint pain. While there are many stem cell providers to be found because of their marketing, choose the center of excellence in Cellular Orthopedics that is evidence based.
Call to schedule a scientific based consultation from an orthopedic surgeon 1 (312) 475-1893.
You may access my web site at www.SheinkopMD.com.
Tags: avascular necrosis, bone lession, bone marrow, Cartilage, cellular orthopedics, clinical study, Growth Factors, IRAP, joint pain, joint replacement, knee pain, knee replacement, meniscus tear, Osteoarthritis, platelets, PRP, regenerative medicine, sports medicine, stem cells, subchondral bone
Jun 22, 2018
My regenerative and restorative Cellular Orthopedics practice is for the most part, evidence based. By that I mean, the outcomes data collected over these past five years regarding the several thousand patients with skeletomuscular afflictions that I have treated with a selection of alternatives using a needle and not a knife is generally based on regenerative and restorative interventions. While not everyone has experienced a dramatic change in symptom relief and functional improvement, many have. The statistical outcomes evidence follows a bell shaped curve with some experiencing immediate improvement as I have in both my hips and knees, while most take several weeks or longer with a continuing improvement up to 18 months post intervention. While it is true that five percent of patients are not satisfied after several years and have gone on to a joint replacement, 95% of my patients are well satisfied and have returned to, or never quit doing what they love.
At the onset of my cellular orthopedic initiative, the interventions were solely based on Platelet Rich Plasma options and Bone Marrow Concentrate; today, our menu of services can be customized so as to meet the needs of all seeking to improve the quality of life and avoid a major surgical procedure. Not only can I concentrate PRP as needed, I can customize the concentration to meet a patient’s particular needs using hemo-analysis. Bone Marrow Concentrate rich in Adult Mesenchymal Stem cells, Platelets, Growth Factors and Precursor Cells is still the foundation of my practice, however for the past year, I am able to offer a Platelet Concentrate derived Growth Factor and Protein Solution option when indicated.
Then there are those whose co-morbidity or prescription medication dependency excludes them from the aforementioned autologous choice of options. As of this upcoming Tuesday, I have acquired an intervention technology that will help patients seeking to a void a total joint replacement who are not candidates for existing regenerative medial offerings. There are many reasons to explain a 5% failure rate including genetic cartilaginous variations, any bell shaped curve will have a small number who don’t pass the final examination. Incidentally, if and when such occurs, I offer another intervention frequently at no charge or certainly at a discounted rate.
Should you want to learn more or schedule a consultation, call
(312) 475-1896. You may visit my web site where you will find the webinar at www.Ilcellulartherapy.com.
Tags: bone marrow, cellular orthopedics, Growth Factors, Hip pain, joint pain, joint replacement, knee pain, Mesenchymal Stem Cell, Osteoarthritis, platelets
Apr 26, 2018
On Wednesday, I completed several bone marrow concentrate procedures for patients with arthritic knees. You will recall that Concentrated Bone Marrow contains living Adult Mesenchymal Stem Cells, Growth Factors, Platelets, Exosomes, Precursor Cells and more allowing for pain relief, improved function and possible regeneration in those afflicted by arthritis. In the afternoon, four patients underwent Autologous Platelet and Growth Factor interventions; two in the hip and two in their knees. An example of the outcome, now four months following intervention in my own knees and hips, I spent last weekend hiking along several spring creeks, fly fishing in Southwest Wisconsin in the morning and planting over 150 Lilly bulbs in the afternoon. Admittedly, I slept well on Saturday and Sunday night but visited the health club on these past Tuesday and Wednesday evenings for my fitness routines.
On Monday, we finalized and edited a manuscript reporting the results of 56 patients with arthritic knees, followed for 2 to 4 years having received Bone Marrow Concentrate. Using the same outcome metrics and statistical tabulation methods I had employed as a joint replacement surgeon, this study is one of the most significant trials ever completed and to be published in Cellular Orthopedics. Our study not only will help determine the indications for a “stem cell” procedure, but also assist in determining how long the benefits will last, and provide a road map for when adjunct or repeat interventions are indicated. Now the physician will be better prepared to help a patient decide between a Total Joint Replacement and a Cellular Orthopedic intervention on an evidence based knowledge.
I am writing this Blog while flying to San Jose, California where I am partaking in advanced training that will allow me to expand my regenerative medicine practice to the low back. Again and again, patients ask as to what I might offer to address low back pain and disc disease now that I have successfully intervened in an arthritic hip or knee. Indications and techniques for addressing the lumbar spine will make up the curriculum enabling me to add discogenic and degenerative arthritic conditions of the low back to my scope of regenerative care by mid May.
To the patient who called, “I heard through the grapevine that it doesn’t work”, you may avoid falling victim to the Fake Stem Cell claims in newspaper ads or via celebrity testimonials; those in amniotic fluid are dead on arrival to you. Seek scientific evidence at (312) 475-1893 or learn more on my web site where you may watch my webinar www.Ilcellulartherapy.com.
Tags: Adult Mesenchymal Stem Cells, arthritic conditions, back pain, cellular orthopedics, Concentrated Bone Marrow, degenerative disc disease, discogenic, Exosomes, Growth Factors, Hip pain, joint pain, knee pain, Osteoarthritis, platelets, Precursor Cells, Regenerative Pain Center, shoulder pain, stem cell therapy

Feb 16, 2018
I am sitting at my computer this morning writing the weekly Blog posting and not attending the IOF meeting taking place today in Broomfield, Colorado; yet I am reporting about the meeting. Instead of attending, I am preparing for a week-long ski adventure with my family next week in Vail, Colorado while trying to catch up in my practice. How is it than possible that I know what is taking place at the meeting? Listed below are five of the 10 ongoing or completed cellular orthopedic clinical trials in which I am a principal investigator or co-researcher. The preliminary and final data resulting from these clinical research initiatives is the outcomes foundation for what is being presented at the IOF podium today and tomorrow.

1) Stem Cell Counts and the Outcome of Bone Marrow Concentrate intra-articular and intra-osseous (subchondroplasty) interventions at the knee for grades 2 and 3 OA. (supported in part by Celling). Ongoing
2) Outcomes of Bone Marrow Concentrate (stem cell, platelet and growth factor) Intervention at the Knee for Grades 2 and 3 OA in 50 patients at 2 to 4 years. (supported in part by Regenexx)
3) Outcomes of Intra-articular Bone Marrow Concentrate versus those of combined Intraarticular and Intraosseous interventions for grades 2 and 3 OA at the knee at one year. (self-funded). Ongoing
4) How does the PRP and Mononucleated cell count affect the outcome of a BMC intervention for grades 2 and 3 Knee OA? (a joint project with Greyledge) Ongoing
5) Safety and Efficacy of Percutaneous Injection of Micro-Fractured Adipose Tissue for grade 4 Osteoarthritic Knees, minimum follow-up of 18 months in 30 patients (supported in part by Lipogems)
I had to prioritize; and since most of the arthritis data being presented is all or in part mine, I already know the subject matter. By staying home, I also found the opportunity to browse “stem cell” websites as suggested by ads in today’s newspapers or introduced by email blasts this week. Wow, a patient acting more like a consumer is really at risk for succumbing to Regenerative Medicine “false news”.
If you want to learn more about the difference between the stem cell purveyors and a legitimate, FDA compliant, evidence based, cellular orthopedics initiative, call to schedule a consultation or to get a second opinion.
You may schedule a visit at (312) 475-1893
You may access my website and watch a webinar at www.ilcellulartherapy.com
Tags: arthritis, bone marrow, Celling, cellular orthopedics, Growth Factors, Hip pain, International Orthopedics Foundation, joint pain, knee arthritis, knee intervention, knee pain, lipogems, Micro-Fractured Adipose, Osteoarthritis, PRP, regenerative medicine, Regenexx, stem cells, Subchondroplasty


