
Feb 11, 2020
This past Sunday morning, I was reading the Chicago Tribune sports section to learn about the pundits’ opinions regarding the basketball game played by Duke and North Carolina on Saturday night, one of the most amazing basketball games I have ever seen, (incidentally, Auburn versus LSU was great, as well) when I came across the Golf Headline “Mickelson’s short game pays off big”. Why my interest? Phil Mickelson has psoriatic arthritis controlled by TNF-Alpha blocker, a Growth Factor. Another golfer whose career was restored by Growth Factors is Tiger Woods. In Woods’ case, he had received Platelet Rich Plasma (PRP) with Growth factors; Platelets produce those biologic agents. Then there is the story of Kobe Bryant, who had traveled to Germany in 2012 to extend his professional career for seven years with Interleukin One Receptor Antagonist Growth Factors recovered from circulating blood. At the time, Bryant travelled to Germany, the treatment he was to receive was not FDA approved in the United States. There is now an innumerable list of professionals and amateurs who have returned to the game or prolonged a career through Growth Factors thanks to the recreational impact of biologics.
If you surf the internet for Regenerative Medicine and Biologic alternatives, you encounter the term “Stem Cells”. The reality is that as of this time, there is no FDA or legal way to avail yourself of Stem Cells alone; the only access is by concentrating your bone marrow and injecting the concentrate into the arthritic joint since there are Adult Mesenchymal Stem Cells in your bone marrow.
Concentrated Platelets, Growth Factors, and Concentrated Bone Marrow have been the regenerative and restorative mainstays in my practice until now.
Looking to the future, because I have been involved with several Cellular Orthopedic Clinical Trials over the past five years, my practice is assisting in the creation of pathways for yet another biologic trial in 2020. Most exciting is the FDA approved Personalized Stem Cell Clinical Trial in which ours is one of seven designated and FDA approved centers. The PSC FDA-approved Clinical Trial was launched in August of last year. In September of 2017, the FDA warned that by November of 2020, all biologics would be reclassified as drugs. Personalized Stem Cells was granted FDA approval to create a drug from your own adipose tissue. The abdominal fat is recovered by Liposuction and processed in a facility that has met all FDA and Good Practice Management governmental requirements. The resultant Stromal Vascular Fraction (Stem Cells recovered from fat) number in the tens of millions and are made available for injection into an arthritic knee. You may learn more at WWW.PSC.Com or on my web site www.SheinkopMD.com. To schedule an appointment or consultation, call (312) 475-1893
Tags: biologics, orthobiologics, recreational impact of biologics
Dec 17, 2019
The progress in the emerging discipline, Orthobiologics, in part, is the result of the FDA and FTC taking on a stronger oversite role; but additionally, the data I gather contributes to these scientific advances. I will elaborate in this blog.
In order to be FDA compliant, the use of stem cells must be autogenous; that is, the cells must come from you. The orthobiologic industry finally complied so those providers with integrity have made available acellular growth factors. Three weeks ago, I learned that is some cases, Medicare and certain insurance companies will preauthorize said usage. Stay tuned on this one as to whether the product works and whether its use is indeed reimbursed.
Last week, I was made aware that the FDA had served notice to Chara Biologics and Liveyon for marketing biologic products that are unapproved. Stay tuned as more bad actors will be shut down.
https://www.fda.gov/news-events/press-announcements/fda-sends-warning-companies-offering-unapproved-umbilical-cord-blood-products-may-put-patients-risk
Biologic Interventions to Avoid Joint Replacements
This past Friday, I received biologic interventions into my right and left hip and my right and left knee. It worked for me two years ago and I believe the biologics will help postpone, maybe avoid joint replacements while allowing me to keep skiing, fly fishing, cycling, and keeping fit. There were no appreciable amounts of stem cells in that which was processed from my own blood and injected. The anticipation is that the Platelets and Growth Factor Proteins will diminish the pain of arthritis, and assist in restoring full motion to my hips and knees, allowing me to continue my recreational profile.
In the presence of a single arthritic knee joint, I would have enrolled in the FDA approved Personalized Stem Cell Clinical Trial. You may learn about the Trial at www.Personalizedstemcells.com or on my web site at www.sheinkopmd.com. In the upcoming months, it is hoped that the FDA will expand approval of the PSC Trial to both knees and eventually, the hips. At this time, there are only two FDA compliant methods of allowing physicians to provide a patient with “stem cells,” one is via your bone marrow and the other via your adipose tissue. In my case, with the several joints involved and given realistic outcome possibilities, I opted for the Growth factor approach. My next step will be the Stem Cell option.
I will be testing my outcomes of the Biologic interventions next week on a bone fishing trip four-five days in Punta Allen, Mexico. While my schedule is full prior to departure, there are still some office openings available on Tuesday, December 31. To schedule an appointment at any time call (312) 475-1893
Tags: biologic intervention, orthobiologics, regenerative medicine, stem cell research, stem cell trials

Dec 5, 2019
Let me think, where did I leave off last week?
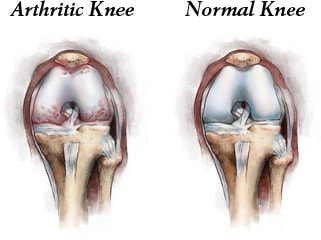
Is there any chance of postponing, or perhaps avoiding a joint replacement, either total or partial? The answer to the question is why I “graduated” from joint replacement to the new discipline of Cellular Orthopedics or Orthobiologics: In the attempt to relieve pain, improve function, restore motion, and stop the progression of osteoarthritis with a needle instead of a knife is based on cells, platelets, and proteins – the latter known as Growth Factors. In order to be FDA compliant, the cells and platelets must be autologous; that is, they must come from you. It is most desirable and effective to use Growth Factors from your bone marrow or circulating blood. Recently, Medicare and some private insurance carriers have pre-authorized amniotic fluid as long as it is produced for pain relief and as a source of Growth Factors but, without stem cells (acellular). Is there the possibility of cartilage regeneration? Or is the goal joint restoration under any set of circumstances?
Orthobiologics Treatment Options
Up until now, other than a Total Joint Replacement, the treatment options for arthritis have been limited in duration of effect, that is palliative. Even a Total Knee Replacement has a limited survivorship or duration of success; indeed, there are some short-term failures and always the risk of a need for revision. In the world of biologics, a repeat intervention is still with a needle, followed by a short-term rehabilitation. Our data indicates that those who repeat a biologic intervention have a better and longer lasting outcome than that following a single injection. Our successes have been focused on blood derived Growth Factors and Platelets and Bone Marrow Concentrate. The latter are autologous (come from you) and FDA compliant.
Returning to the beginning of the Orthobiologics Informed Consent message, I haven’t forgotten about my nephew; the previously very athletic one with the Grade 2 to 3 Osteoarthritis of the knee. The therapeutic recommendation is to start with an injection of growth factors and Platelets obtained from his circulating blood. The benefits should be realized in less than six weeks and last two to three years. After that time, he could repeat the initial injection process or advance to a Bone Marrow Concentrate intervention. Bone marrow contains stem cells introducing regenerative potential a swell as providing a wealth of Growth Factors. Both blood derived platelets with Growth Factors and Bone Marrow Concentrate are Evidence Based therapies.
Recently, a third option became available to me; Stromal Vascular Fraction or Stem cells derived from your adipose tissue. At this time, there are seven centers in the United States, approved by the FDA, participating in the Personalized Stem Cell Trial; I am one of the investigators. The PSC Trial is that of a drug created from your adipose tissue, the latter harvested from your own fat and processed under very stringent governmental controls, processed and injected into your knee. To learn more about the PSC Trial visit www.PersonalizedStemCells.com. You may visit my web site at www.sheinkopmd.com to become familiar with my treatment options and participate in my webinars. Your consultation may be scheduled by calling (312) 475-1893.
Tags: cellular regeneration, orthobiologic consent, orthobiologics, stem cell trials
Jan 10, 2019
Nonobstructive meniscal tears
There is increasing evidence to suggest that patients with meniscal tears at the knee that do not cause “clunking”, giving way, or locking; hence nonobstructive, may benefit from Cellular Orthopedic intervention coupled with physical therapy. Previous studies involving patients over 45 years of age comparing arthroscopy with physical therapy for nonobstructive meniscal tears as seen on an MRI justify an initial conservative approach; but patient satisfaction may require 24 months to achieve. For those patients who undergo arthroscopic surgery, there is a significant increased risk of repeat knee surgery. In our practice, those patients electing to use the Physical Therapy option without surgery but with a Cellular Orthopedic intervention minimized the length of time needed to return to full activity.
Number of stem cells in amniotic fluid
The functionality of stem cells in amniotic fluid as sold today is a myth. Research shows that 250cc of fresh C-section delivered amniotic fluid, when introduced immediately into culture, only yields 40 stem cells. This means there are 0.16 stem cells per 1 cc of full-term amniotic fluid. Scientific literature referred to by the amniotic fluid marketing forces is based on amniotic fluid collected early in pregnancy.
Acetabular Labral Tear
A hip (acetabular) labral tear is damage to cartilage and tissue in the hip socket. In some cases, it causes no symptoms. In others it causes pain in the groin. Just because a tear is seen in the hip labrum on an MRI, it does not mean the tear is necessarily the cause of the pain. Before initiating treatment, the orthopedic surgeon must exclude that an underlying arthritic condition within the hip is not the real pain generator. More recently recognized is predisposition for a tear in those with abnormal acetabular architecture.
On biologics for knee osteoarthritis
Orthobiologics may become a mainstream treatment for knee osteoarthritis. While Platelet-rich plasma and hyaluronic acid injections are the most established biologics-based treatments for knee osteoarthritis so far, it’s not too early to make confident use of stem cells. At the same time, I must continually warn patients to be particularly careful about claims for these substances. All recommendations for intervention must be FDA compliant and evidence based. (To learn about my contributions to the cellular orthopedic scientific evidence, visit www.sheinkopmd.com. Under the information bullet on the top, you will find published articles)
Eventually, I believe the science and FDA will triumph over quackery and orthobiologics will become an essential part of every knee surgeon’s armamentarium. Available orthobiologics, include:
- Hyaluronic acid
- Platelet-rich plasma
- Cytokine modulation
- Stem Cells
- Exosomes
- Adipose tissue
To learn more or to schedule an evidence based consultation, call (312) 475-1893
Tags: adipose tissue, amniotic fluid, biologics, bone marrow, cytokine modulation, Exosomes, fat, hyaluronic, joint, labral tear, Micro-Fractured Adipose, orthobiologics, Osteoarthritis, pain, Platelet Rich Plasma, PRP, renovation, stem cell, torn meniscus
Oct 18, 2018
We are speaking of stem cell therapy integrated with clinical research, and the resultant evidence-based stem cell intervention. Osteoarthritis is becoming more prevalent as I am seeing younger patients with arthritis as a consequence of sporting injuries such as ACL tears. The baby boomer population is experiencing accelerated onset of arthritis; their joints are prematurely aging in large numbers. At the same time, the master population is aging and living longer. As a result, I continually research biologic interventions to best address the ever-increasing number of those effected.
Why should a patient choose an orthopedic surgeon to manage their Osteoarthritic related symptoms and functional impairment? Our world is evidence based.
Study Observes Better Outcomes for OA Patients Treated by an Orthopaedic Specialist
In a retrospective study published online in BMC Musculoskeletal Disorders, shoulder osteoarthritis (OA) patients received faster and more invasive treatment when they received a new diagnosis from an orthopaedic specialist (OS) versus a nonorthopaedic physician (NOP). Patients with shoulder OA (n = 572) received care from either an OS (n = 474) or NOP (n = 98) on the date of their index shoulder visit. OS patients received their first treatment significantly quicker than the NOP cohort (16.3 days versus 32.3 days, respectively). The OS group also had higher rates of operative treatment within one year following their initial visit.
Study: Patients Report Similar Improvements for Nonobstructive Meniscal Tear with PT and Early Surgery
Physical therapy (PT) may not be inferior to early operative treatment of arthroscopic partial meniscectomy (APM) for improving knee functionality in patients with nonobstructive meniscal tears, according to a study published online inJAMA. The randomized clinical trial included 321 patients with nonobstructive meniscal tears aged 45 to 70 years who were treated at nine hospitals in the Netherlands between July 17, 2013, and Nov. 4, 2015. Patients were treated with APM (n = 159) or a predefined PT protocol (n = 162) that included 16 exercise therapy sessions over eight weeks. PT sessions focused on coordination and closed kinetic chain strength exercises. At 24-month follow-up, knee functionality in the PT group improved by 20.4 points compared to 26.2 points in the APM group. The difference did not exceed the noninferiority margin.
In order to maximize the benefits, Orthobiologics, that is stem cell therapy must be integrated with clinical research, and the resultant evidence-based stem cell intervention followed long term. In my practice, I am researching biologic interventions to address the ever-increasing number of those effected, not one and done. To learn more or schedule a consultation, Call (312)475-1893. You may visit my web site and read my blogs at www.sheinkopmd.com
Tags: ACL tear, arthritis, Cartilage, cellular orthopedic, joint pain, joint replacement, knee pain, MCL tear, meniscus tear, menisectomy, orthobiologics, orthopedic surgen, Osteoarthritis, Physical Therapy, PRP, regenerative medicine, stem cell



