Apr 16, 2018
My team dedicates an inordinate amount of time answering questions and attempting to clarify the misunderstanding of patients when it comes to Platelet Rich Plasma; actually, the entire subspecialty of “Stem Cell Therapy” but let’s start with PRP. As an orthopedic surgeon who introduced Cellular Orthopedics to the Midwest five years ago, I am in a unique position to help define the problem. Does PRP have a role in treating a painful or injured part of the musculoskeletal system? In an attempt to help clarify misconceptions and better define the term Platelet Rich Plasma, I sat down and wrote this Blog.
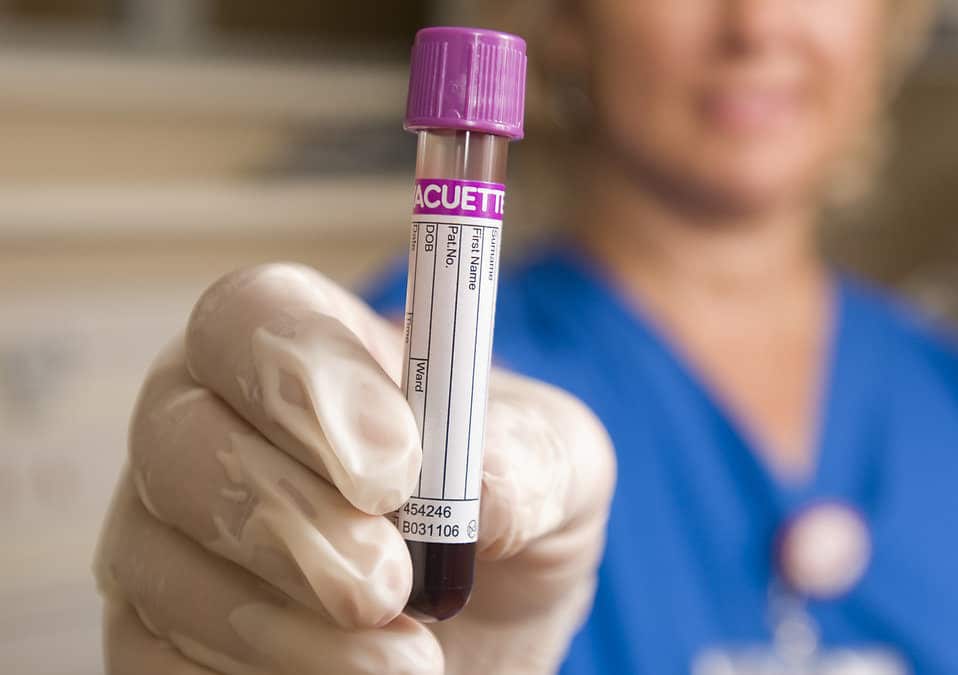
Platelets circulating in the blood play a fundamental role in blood clotting and are a natural source of growth factors. Platelet rich plasma (PRP), also termed autologous platelet gel, plasma rich in growth factors (PRGF), platelet concentrate (PC), is essentially an increased concentration of (autologous) your platelets suspended in a small amount of plasma after centrifugation. Although it is not exactly clear how PRP works, laboratory studies have shown that the increased concentration of growth factors in PRP can potentially speed up the healing process.
The amount of PRP necessary to achieve the intended biologic effects still remains unclear.; but we know PRP contains growth factors in high concentrations. Precise predictions of growth factor levels based on the platelet counts of whole blood or PRP are limited. In our office, we use a hemocytometer to count platelets and the different white blood cells contained in the preparation. Knowing there are different sources for growth factors (platelets, leukocytes, plasma), we assume the higher number of platelets and leukocytes counted in the hemocytometer, the higher the concentration of growth factors in the preparation. Treatments using these autologous platelet growth factors are an important reason to improve methods for isolating platelet-rich plasma (PRP) and that is why I am involved in an initiative to correlate counts with clinical outcomes.
PRP proponents assert that concentrated Platelet Rich Plasma fails to successfully treat symptoms in some cases because of differences in PRP formulation. There is no standardization thus leading to variables, such as PRP preparation methods, the amount of PRP injected, and the frequency of injections. These inconsistencies result in issues raised by patients: “PRP didn’t work for me” and “I had 15 PRP injections to my knee and I still have pain”. In addition to studying the numbers and monitoring results, I am involved with initiatives to filter and concentrate the growth factors in PRP so as to improve outcomes as well.
1)Platelet Rich Plasma
2)Concentrated Platelet Rich Plasma
3)Concentrated Stem Cell Plasma
4)Autologous Platelet and Growth Factor Concentrate
When you call (312- 475- 1893) to schedule a consultation or watch my webinar at www.Ilcelulartherapy.com, you will avail yourself of the aforementioned Platelet Rich Plasma treatment options in addition to our entire Cellular Orthopedic menu of regenerative care.
Tags: autologous injection, autologous platelet and growth factor concentrate, hemocytometer, injection, leukocytes, Platelet Rich Plasma, PRGF, PRP, Stem Cell Plasma
Feb 2, 2018
For those who may have missed it, I was featured Monday night in a Fox 32 news report presented by Fox News investigative reporter Sylvia Perez.
http://www.fox32chicago.com/health/customers-warn-that-doctors-are-scamming-patients-with-fake-stem-cell-claims
Regular readers of my Blog are aware of the opinions I have frequently expressed regarding the charlatans and camp followers that have taken advantage of the regenerative medicine marketplace promising to cure arthritis, Alzheimer’s, Alopecia, ALS, Autism, and every malady known to mankind finally ending at the letter Z. They don’t exclude spinal cord injury, residuals of stroke nor ED while they are at it. The message regarding what stem cells can do is found in newspaper ads, television commercials and radio spots, the latter in the Chicago listening area by a well-known sports announcer. Either attend a seminar or make an appointment for treatment; they will cure your disease, eliminate pain and do away with your suffering. “Call now to schedule an appointment”.
For a free lunch and without an evaluation or examination, you can undergo an amniotic fluid intervention that is “regenerative” as it is claimed, at a cost in the neighborhood of $5,000. I have been involved in amniotic fluid clinical trials for four years underwritten by the largest provider of amniotic fluid in the nation; and our first statement to participants in these clinical trials, without charge for the injectate, is that there are no living stem cells in the amniotic fluid once processed, sterilized, frozen and fast thawed for usage. Hold on, there is more. On September 16, 2017 the FDA published mandatory guidelines: any and all regenerative agents must be autologous and homologous. In plain speak the injectate must come from the same patient and be used as nature intended. Stem cells from donor sources are not compliant.
Featured in the Fox News special report are two patients. One had undergone a complete medical history, physical examination and skeletomuscular evaluation prior to his Cellular Orthopedic intervention enjoying a marvelous outcome; the other, an amniotic fluid injection into his knee without any prior evaluation or preparation and an awful end result. You may watch the actual report by clicking on that underscored above.
One of the standard of practice methodologies in which we take great pride and which I believe separates us from the madding crowd of regenerative medicine camp followers and charlatans; is our evidence based cellular orthopedic approach. In preparation for a scientific podium presentation in two weeks, we are collating our outcomes data at one year for patients who underwent a combined intraarticular (into the knee) and intraosseous (into the subchondral bone) autologous bone marrow and growth factor intervention for osteoarthritis grades two and three. At six weeks, we recorded a 22% improvement in pain relief; 42% at six months, and 89% at 12 months. In future blogs, I will breakdown the outcomes data further and expand on our documented outcomes based on our several cellular orthopedic options.
To learn more, you may review my web site and watch my webinar at www.ILcellulartherapy.com
You may schedule a consultation by calling (312) 475-1893
Tags: autologous proten, bone marrow, C-SCP, cellular orthopedics, Clinical Studies, Clinical Trial. Mitchell B. Sheinkop, Concentrated Stem Cell Plasma, FDA, Growth Factors, Interventional Orthopedics, joint replacement, Knee Pain Relief, Osteoarthritis, Platelet Rich Plasma, Subchondroplasty

Dec 27, 2017
Or as Dan Brown’s Origin explores, “Where did we come from, where are we going?”
It seems customary to make predictions at this time of year; but I want to begin by looking back four decades. It is more than reminiscence. Until managed health care was introduced into the marketplace undermining the doctor-patient relationship, a patient would make health care decisions for the most part based on the trust and confidence established with the family physician. Then came what was promoted as New Horizons of Health Care; mainly insurance based decision making. Hailed as the means of controlling spiraling health care costs, the doctor-patient relationship was no longer primary. The model changed health care decision making from a physician to a non-professional employee sitting in front of a computer and determining your care based on a cost containment paradigm. The next step in devolution was the appearance of Web MD and the like were in everyone thought they could become a physician and expert in some aspect of health care by simply clicking on a mouse. No need now for a physician or expert any longer; why bother with someone educated in medical school and struggling through a lengthy residency and then fellowship? Then appeared the next step in Gulliver’s Travels, further devolution or the opportunity for camp followers and charlatans to exploit the belief that marketing would outweigh evidence and science in a patient’s decision-making process.
Unfortunately, the FDA is only now beginning to police these amniotic fluid stem cell purveyors offering false hope. We have experienced a study process of lowering standards, further eroding norms, and peddling fiction. Where are we going; where should you turn for orthopedic care?
Today, I am undergoing a cellular orthopedic intervention on my knees so as to assure my best possible performance when I fly fish, ski, cycle, and maintain my fitness profile in 2018. The process is an autologous therapy (from me to me), that I have wanted to bring to the United States since Kobe Bryant and many more travelled to Dusseldorf, Germany, to have completed over seven years ago. The promise is to do more than treat my knee pain; rather I hope to slow the progression of my Osteoarthritis with this cell-concentration system.
The poet Robert Browning wrote a poem, Rabbi Ben Ezra, often cited as we age and I will quote the opening stanza here:
“Grow old along with me! The best is yet to be”
You may read the entirety at the Poetry Foundation website. You may watch my webinar on my web site at www.Ilcellulartherapy.com. Call to schedule appointment (312) 475-1893.
Read this Blog to monitor my response to treatment
Tags: arthritis, autologous therapy, Clinical Trial. Mitchell B. Sheinkop, IRAP, Knee Pain Relief, Kobe Bryant, Mesenchymal Stem Cell, Osteoarthritis, Platelet Rich Plasma
Dec 6, 2017
On November 16, 2017, The FDA posted definitive guidelines concerning what meets minimal manipulation rules and regulations and what is accepted under the practice of medicine guidelines in the specialty of Regenerative Medicine. The FDA further restated the requirement that regenerative medicine be governed by homologous use. As I interpret the guidelines there are winners and losers:
Winners
Physicians who use compliant regenerative therapies:
- Amniotic fluid without stem cells
- Blood-derived preparations (e.g., PRP, PPP)
- Bone marrow aspirate
Losers
Physicians who use non-compliant regenerative therapies:
- Adipose tissue-derived materials obtained by enzymatic digestion
- Amniotic fluid with cells Cord blood derived materials (non-autologous treatments)
- Stem Cell Clinics that advertise about using amniotic fluid as a source of stem cells and regenerative therapy along with those clinics that treat everything from alopecia to ALS to arthritis
You might ask how is that different from the current situation? First of all, the FDA Commissioner has stated in press releases that the FDA is going to go after bad actors. The Cures Act provided for increased funding to the FDA, which we suspect the Commissioner will use in part to go after the bad actors. Also, the FDA wrote in their Guidance on Minimal Manipulation and Homologous Use that “healthcare providers” need to pay attention. We have never seen them explicitly refer to the doctors and clinics providing regenerative medicine. Finally, the FDA indicated that there would be a transition period (3 years) during which manufacturers would need to enter the RMAT program to get their non-compliant products properly approved; or else. And the reason that there could be teeth in the “or else” is that the FDA will get lots of fees from all of the non-compliant products entering the RMAT program.
Last of all, what the FDA did not address as part of consumer protection; but what I incorporate in my daily practice is evidence based intervention.
Now that you are better informed and have an idea as to the laws governing our regenerative medicine marketplace, stay away from the Charlatans and Camp Followers. Then take the next step and ask your physician for the Outcomes Evidence on which a regenerative intervention for your arthritic joint is based before undergoing a procedure. To better understand that evidence call for (312) 475 1893 to set up a consultation
You may watch my webinar by accessing my web site www.ilcellulartherapy.com.
* Minimal Manipulation and Homologous Use
Tags: adipose tissue, Bone Marrow Concentrate, Clinical Studies, Clinical Trial. Mitchell B. Sheinkop, FDA, Hip Replacement, Interventional Orthopedics, joint replacement, Mesenchymal Stem Cell, Minimal Manipulation and Homologous Use, Orthopedic Care, Orthopedic Surgeon, Platelet Rich Plasma, PRP, regenerative therapies, stem cells
Nov 29, 2017
I am being forthright; based on my review of data, while 80% or more of my patients continue to enjoy
satisfactory outcomes at four years or more following a stem cell intervention, there are those whose
symptoms and functional limitations recur. Please be aware that when I undertake the care and
treatment of a patient with a symptomatic and function limiting joint, it is with the notion of
regeneration and long-term benefit. It doesn’t always happen; there are may possible explanations.
Most important though is the need to identify possible causes of potential failure at the beginning, and
that is why we have recommendations before and after a procedure as to how to manage alcohol, diet,
supplements and a rehabilitation protocol. We also review your past medical history to identify any
possible indication that your stem cells have been adversely affected by co-morbidity or prior
treatments.
Assume if you will that you adhered to the initial pre-and post-intervention protocol but now returned
to my office months or years later with recurring symptoms. First and foremost is an updated medical
history and physical examination. That is followed by repeat images including X-rays and an MRI.
Mechanical progression of joint injury may result from aggravation of the preexisting damage by
subsequent trauma. Then there is the reality of identifying new processes within or adjacent to the joint.
This morning, I returned the phone call of a southwest Wisconsin dairy farmer; not the same patient I
wrote about last week. He has been a patient for over four years with a full restoration of work related
activities and recreational pursuits following several regenerative interventional options. After three
hours of basketball, three weeks ago, his knee pain returned. I called him back while he was milking his
cows and it was the first time I have been “mooed” at over a cell phone. I requested that the patient
update his X-rays, MRIs and then allow me to reevaluate him. A repeat stem cell intervention with a
more advanced technology, a subchondroplasty in addition to the stem cell intervention of his joint?
The recommendations will be based on an updated evaluation. In my practice of cellular orthopedics, it
isn’t one and done. Additionally, some of the more advanced techniques are being covered in part by
health care insurance
If you want to learn more, call for an appointment (312)475 1893
You may access my web site at www.Ilcellulartherapy.com and watch my webinar
After I completed writing this Blog, I opened the Bone and Joint Newsletter.
Lead article: Study Suggests Knee Replacement Be Reserved for Those More Severely Affected by Osteoarthritis. A recent analysis found that the current practice of TKR as performed in the USA had minimal effects on quality of life and quality adjusted life years
Tags: arthritis, Benefits and Risk, bone marrow, Bone Marrow Concentrate, Clinical Studies, Clinical Trial. Mitchell B. Sheinkop, Concentrated Stem Cell Plasma, Growth Factors, Hip Replacement, Interventional Orthopedics, Knee Pain Relief, Mesenchymal Stem Cell, Orthopedics, Osteoarthritis, Pain Management, Platelet Rich Plasma, regenerative medicine, stem cell treatment

